Phenolphthalein Indicator w/ Dropper Bottle
$9.99 – $34.99
Technical Specifications
- General Information
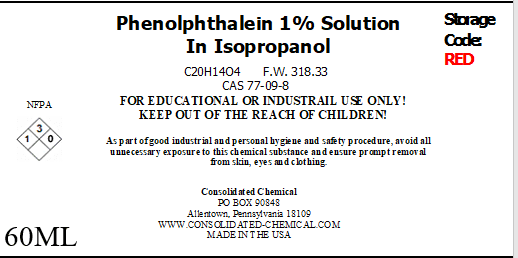
- Product Name: Phenolphthalein 1% Solution
- Chemical Name: Phenolphthalein
- Chemical Formula: C₂₀H₁₄O₄
- CAS Number: 77-09-8
- Appearance:
- Clear, colorless to slightly pale liquid in acidic and neutral environments.
- Turns pink/magenta in basic environments (pH > 8.2).
Composition
- Phenolphthalein Concentration: 1% (w/v)
- Solvent: Typically ethanol, methanol, or a water-alcohol mixture.
- Additives: None (pure indicator solution).
Physical Properties
- Density: ~0.79–0.81 g/cm³ (depending on alcohol content).
- Boiling Point: Dependent on solvent used (e.g., ~78 °C for ethanol).
- Melting Point (of pure phenolphthalein): 258–263 °C.
- pH Transition Range:
- pH < 8.2: Colorless.
- pH 8.2–10.0: Gradual transition to pink.
- pH > 10.0: Bright pink/magenta.
- Solubility:
- Fully soluble in alcohol-based solvents.
- Slightly soluble in water when undissolved.
Documents: