Ammonium Hydroxide 29% – Premium Aqueous Solution
$14.99 – $29.99
General Information
- Chemical Name: Ammonium Hydroxide
- Synonyms: Aqueous Ammonia, NH₄OH
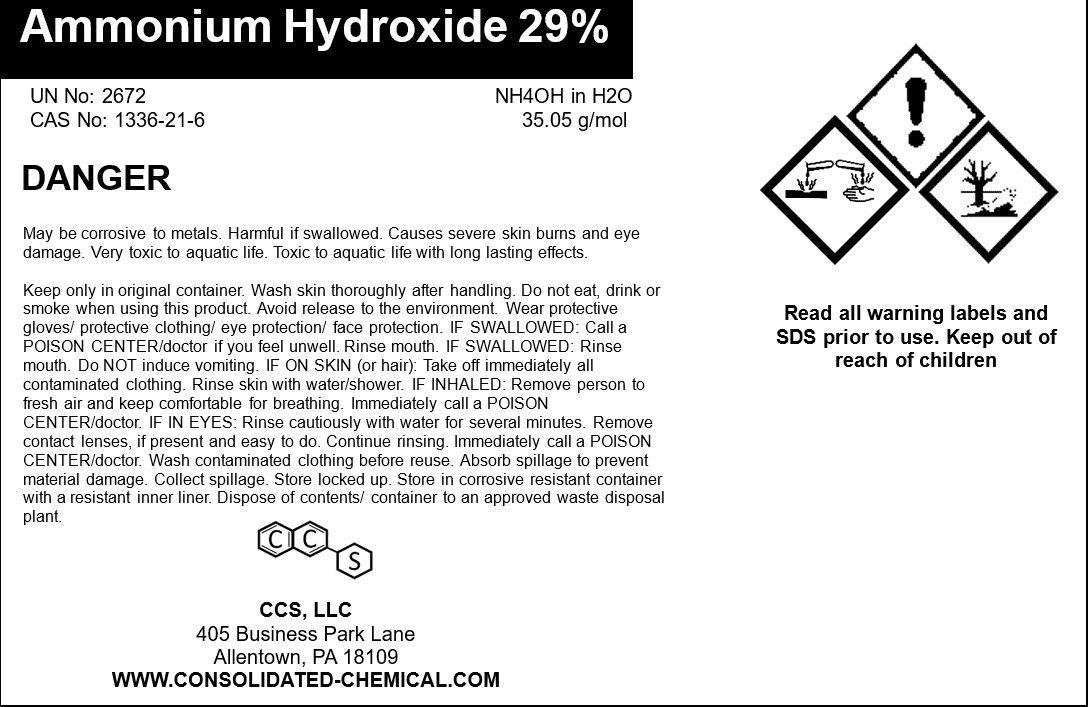
- CAS Number: 1336-21-6
- EC Number: 215-647-6
- Molecular Formula: NH₄OH (aqueous solution)
- Molecular Weight: 35.05 g/mol
- Concentration: 29% Ammonia by weight
Physical Properties
- Appearance: Clear, colorless liquid
- Odor: Strong, pungent ammonia odor
- Density: ~0.90 g/cm³ at 20°C
- Boiling Point: ~38°C (depends on pressure)
- Freezing Point: -72°C
- pH: Highly alkaline, ≥ 11.6
- Solubility: Fully miscible in water
Chemical Properties
- Stability: Stable under normal storage conditions. Can decompose into ammonia gas if heated or exposed to acidic conditions.
- Reactivity:
- Reacts with acids to form ammonium salts.
- May corrode certain metals such as zinc, copper, and aluminum.
Documents:
Description
Our Ammonium Hydroxide 29% is a high-quality, concentrated aqueous ammonia solution widely used in cleaning, industrial, and laboratory applications. Its high alkalinity and versatility make it a preferred choice for chemical processes, pH adjustments, and effective cleaning solutions. Whether for industrial-grade cleaning or laboratory research, this product ensures reliability and consistent performance.
Applications of Ammonium Hydroxide 29% – High-Quality Aqueous Solution
Industrial Applications
- Water Treatment:
- Used to adjust pH levels in industrial and municipal water treatment systems.
- Chemical Manufacturing:
- Acts as a reagent and intermediate in the production of fertilizers, plastics, and dyes.
- Metal Processing:
- Used in electroplating and metal cleaning processes to remove contaminants.
Cleaning Solutions
- Household Cleaners:
- A common ingredient in glass cleaners, floor cleaners, and degreasers.
- Industrial Cleaning:
- Effective for removing grease, oil, and residue from equipment and surfaces.
Laboratory Applications
- Analytical Chemistry:
- Used in titrations and for the preparation of ammonium salts.
- Buffer Preparation:
- Creates pH-stable solutions for research and analytical procedures.
Textile and Dye Industry
- Fabric Treatment:
- Used in bleaching, dyeing, and treating fabrics for color retention and quality.
Agriculture
- Fertilizer Production:
- Provides a nitrogen source for ammonium-based fertilizers, enhancing crop growth.
- Soil pH Adjustment:
- Neutralizes acidic soils for improved plant health.
Food and Beverage Industry
- Food Processing:
- Used in limited applications as a pH regulator and cleaning agent for food production equipment.
Electronics Industry
- Etching and Cleaning:
- Removes oxides and residues from semiconductor surfaces and electronic components.
Pulp and Paper Industry
- Processing Aid:
- Adjusts pH during the pulping and bleaching stages.
Storage Instructions:
- Temperature: Store in a cool, dry, and well-ventilated area at temperatures between 5°C and 25°C (41°F–77°F).
- Avoid Heat and Sunlight: Keep away from direct sunlight, heat sources, and open flames to prevent decomposition and pressure buildup.
- Sealed Containers: Ensure containers are tightly closed to minimize evaporation and prevent contamination.
- Ventilation: Store in areas with adequate ventilation to avoid vapor buildup.
- Compatibility: Keep away from acids, oxidizing agents, and reactive metals (e.g., aluminum, zinc).
Handling Instructions:
- Personal Protective Equipment (PPE):
- Always wear chemical-resistant gloves, safety goggles, and a protective apron to prevent contact with skin and eyes.
- Use a respirator if working in areas with poor ventilation.
- Minimize Exposure:
- Avoid inhaling vapors or mist.
- Use in a fume hood or well-ventilated space.
- Safe Transfer:
- Use appropriate tools to avoid spills or splashes. Use non-metallic equipment to prevent corrosion.
- Spill Management:
- For small spills: Neutralize with a weak acid (e.g., vinegar) and absorb with inert material like sand.
- For large spills: Contain and collect the liquid for proper disposal.
- Hygiene Practices:
- Wash hands and exposed skin thoroughly after handling.
- Avoid eating, drinking, or smoking in the work area.
Disposal:
- Dispose of unused product and empty containers in compliance with local, state, and federal regulations.
- Do not pour into drains, waterways, or soil.
Additional information
| Size | 100mL (3.3 Fl Oz), 250mL (8 Fl Oz), 500mL (16 Fl Oz), 1000mL (32 Fl Oz) |
|---|
Be the first to review “Ammonium Hydroxide 29% – Premium Aqueous Solution” Cancel reply
Related products
-

Potassium Carbonate – Food Grade (E501)
$24.99 – $39.99 Select options This product has multiple variants. The options may be chosen on the product page -

1-Octanol (Alcohol C-8) Premium Aroma Fragrance Compound
$12.00 – $95.00 Select options This product has multiple variants. The options may be chosen on the product page -

Silver Nitrate – Premium High-Purity Grade
$19.99 – $220.00 Select options This product has multiple variants. The options may be chosen on the product page -

Methylene Blue Stain/Dye High Purity, Poly Bottle, 10g-50g
$12.50 – $34.00 Select options This product has multiple variants. The options may be chosen on the product page
SKU: N/A
Categories: Bases (9), Food Additive, Industrial Chemical, pH Regulator, Water Treatment
Tags: 29%, ammonia water 29%, Ammonium, Ammonium Hydroxide, Ammonium Hydroxide 29%, ammonium hydroxide Alibaba, ammonium hydroxide alternative applications, ammonium hydroxide alternative uses, ammonium hydroxide Amazon, ammonium hydroxide analytical reagent, ammonium hydroxide as a food additive, ammonium hydroxide B2B marketplace, ammonium hydroxide biodegradable, ammonium hydroxide boiling point, Ammonium Hydroxide Bulk Supplier, ammonium hydroxide CAS 1336-21-6, ammonium hydroxide chemical applications, ammonium hydroxide chemical reagent, ammonium hydroxide concentration, ammonium hydroxide corrosive liquid, ammonium hydroxide custom formulations, ammonium hydroxide density, Ammonium Hydroxide Distributor, ammonium hydroxide e-commerce, ammonium hydroxide eBay, ammonium hydroxide eco-friendly, ammonium hydroxide environmental applications, ammonium hydroxide environmental impact, ammonium hydroxide extended stability, ammonium hydroxide flash point, ammonium hydroxide food-grade, ammonium hydroxide for ammonia production, ammonium hydroxide for chemical processing, Ammonium Hydroxide for Chemical Synthesis, Ammonium Hydroxide for Cleaning, Ammonium Hydroxide for Fertilizers, Ammonium Hydroxide for Food Processing, Ammonium Hydroxide for Glass Cleaning, Ammonium Hydroxide for Household Cleaning, ammonium hydroxide for industrial cleaning, Ammonium Hydroxide for Industrial Use, Ammonium Hydroxide for Laboratories, Ammonium Hydroxide for Metal Cleaning, Ammonium Hydroxide for pH Control, ammonium hydroxide for process engineering, ammonium hydroxide for research, Ammonium Hydroxide for Sale, Ammonium Hydroxide for Surface Preparation, Ammonium Hydroxide for Textiles, ammonium hydroxide for wastewater treatment, Ammonium Hydroxide for Water Treatment, ammonium hydroxide formula, ammonium hydroxide freezing point, ammonium hydroxide GHS classification, ammonium hydroxide global demand, ammonium hydroxide handling, ammonium hydroxide high concentration, ammonium hydroxide high-purity, ammonium hydroxide import export, ammonium hydroxide in acid neutralization, ammonium hydroxide in agriculture, ammonium hydroxide in ammonium salt manufacturing, ammonium hydroxide in baking, ammonium hydroxide in biopesticides, ammonium hydroxide in bulk, ammonium hydroxide in chemical synthesis, ammonium hydroxide in cleaning products, ammonium hydroxide in cooling systems, ammonium hydroxide in dairy processing, ammonium hydroxide in degreasers, ammonium hydroxide in detergents, ammonium hydroxide in electroplating, ammonium hydroxide in fertilizers, ammonium hydroxide in gas scrubbing, ammonium hydroxide in herbicides, ammonium hydroxide in industrial cleaners, ammonium hydroxide in industrial production, ammonium hydroxide in laboratory experiments, ammonium hydroxide in meat processing, ammonium hydroxide in medical applications, ammonium hydroxide in metal processing, ammonium hydroxide in organic synthesis, ammonium hydroxide in pesticides, ammonium hydroxide in petroleum refining, ammonium hydroxide in pH control, ammonium hydroxide in pharmaceutical manufacturing, ammonium hydroxide in rubber manufacturing, ammonium hydroxide in textile processing, ammonium hydroxide industrial applications, ammonium hydroxide industrial sales, ammonium hydroxide industrial-grade, ammonium hydroxide industry insights, ammonium hydroxide international distribution, ammonium hydroxide laboratory-grade, ammonium hydroxide long shelf life, ammonium hydroxide low impurity, ammonium hydroxide manufacturer, ammonium hydroxide market trends, ammonium hydroxide molecular weight, ammonium hydroxide MSDS, ammonium hydroxide odor, ammonium hydroxide online ordering, ammonium hydroxide oxidation resistance, ammonium hydroxide pH, ammonium hydroxide price, ammonium hydroxide procurement, ammonium hydroxide quality assurance, ammonium hydroxide raw material, ammonium hydroxide REACH compliance, ammonium hydroxide regulatory compliance, ammonium hydroxide safety data, ammonium hydroxide safety guidelines, ammonium hydroxide SDS, ammonium hydroxide shipping worldwide, ammonium hydroxide solubility, Ammonium Hydroxide Solution, ammonium hydroxide sourcing, ammonium hydroxide storage, ammonium hydroxide technical specifications, ammonium hydroxide trade, ammonium hydroxide transportation, ammonium hydroxide vapor pressure, ammonium hydroxide Walmart, Ammonium Hydroxide Wholesale, Aqua Ammonia, Aqueous Ammonia, aqueous ammonia 29%, Buy Ammonium Hydroxide Online, High-Purity Ammonium Hydroxide, High-Quality Ammonium Hydroxide, Hydroxide, Industrial Ammonium Hydroxide, Laboratory Grade Ammonium Hydroxide, Reliable Ammonium Hydroxide







Reviews
There are no reviews yet.