Phenolphthalein Indicator w/ Dropper Bottle – Lab Grade
$9.99 – $34.99
Chemical Information
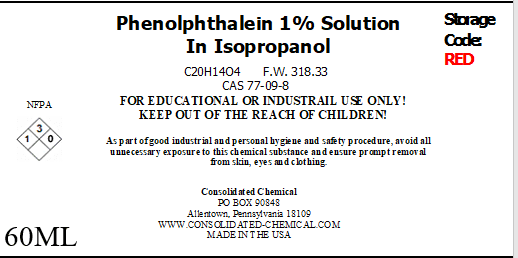
- Chemical Name: Phenolphthalein Indicator Solution
- Common Names: Phenolphthalein, pH Indicator, Acid-Base Indicator
- CAS Number: 77-09-8
- Molecular Formula: C₂₀H₁₄O₄
- Molecular Weight: 318.32 g/mol
- Appearance: Clear, colorless liquid
- Odor: Odorless
- Concentration: Typically 0.5% or 1% w/v in ethanol or water
- Purity: Laboratory Grade
Physical & Chemical Properties
- pH Transition Range: Colorless (pH < 8.2) to Pink (pH 8.2 – 10.0)
- Solubility: Soluble in alcohol, slightly soluble in water
- Boiling Point: ~100°C (212°F) (aqueous solution)
- Density: ~1.0 g/cm³
- Reactivity: Stable under normal storage conditions
Mechanical & Stability Properties
- Combustibility: Non-combustible (aqueous solution), flammable if ethanol-based
- Corrosiveness: Non-corrosive
- Stability: Light-sensitive; store in amber bottles
Documents:
Description
Phenolphthalein Indicator Solution is a high-purity pH indicator widely used in acid-base titrations, laboratory analysis, medical diagnostics, and educational experiments. It is a colorless compound in acidic solutions but turns pink to fuchsia in alkaline conditions, making it an essential reagent in analytical chemistry, water testing, and industrial quality control.
Applications of Phenolphthalein Indicator:
1. Laboratory & Analytical Applications
- Acid-Base Titrations: Used as a pH indicator in acid-base titration reactions, turning colorless in acidic solutions and pink in alkaline solutions.
- pH Measurement: Provides quick and accurate identification of acidic or basic solutions in laboratories.
- Analytical Chemistry: Used in chemical analysis, research, and industrial quality control.
- Water Quality Testing: Helps determine alkalinity and acidity in water treatment plants.
- Forensic Science: Utilized in blood detection tests and forensic investigations.
2. Industrial & Manufacturing Applications
- Chemical Processing: Essential in monitoring pH changes in chemical manufacturing processes.
- Soap & Detergent Manufacturing: Used to measure and adjust pH in formulations.
- Textile & Dyeing Industry: Helps control pH levels in textile processing and dyeing applications.
- Food & Beverage Industry: Used in food safety testing and pH regulation.
- Petrochemical Industry: Applied in acid neutralization and process optimization.
3. Medical & Pharmaceutical Applications
- Medical Diagnostic Testing: Used in urine pH tests, gastric acid analysis, and intestinal health monitoring.
- Pharmaceutical Research: Utilized in drug formulation and stability testing.
- Biochemical Studies: Applied in enzyme research and protein interaction studies.
4. Educational & Research Applications
- Science Education: Widely used in high school and university chemistry labs for teaching acid-base reactions.
- Chemical Demonstrations: Popular for science experiments demonstrating pH changes and color transitions.
- STEM Learning Kits: Included in chemistry lab kits for interactive learning.
5. Environmental & Water Treatment Applications
- Wastewater Treatment: Used in monitoring and adjusting the pH of industrial effluents.
- Soil Testing & Agriculture: Helps determine soil pH levels for optimal crop growth.
- Air Pollution Studies: Utilized in acid rain research and CO₂ absorption testing.
6. Forensic & Safety Applications
- Blood Detection Tests: Used in forensic investigations for detecting latent blood stains.
- Corrosion Testing: Helps assess the impact of acidic or basic environments on metals.
- Firefighting Foam Testing: Used in chemical analysis of firefighting foams and extinguishing agents.
Storage
- Store in a cool, dry, and well-ventilated area.
- Keep in light-resistant (amber) bottles to prevent degradation.
- Keep away from heat, sparks, and open flames if ethanol-based.
Handling
- Use protective gloves, safety goggles, and lab coats when handling.
- Avoid contact with skin and eyes; can cause irritation.
- Use in a well-ventilated environment or fume hood.
Safety
- May cause irritation upon contact with skin or eyes.
- Flammable if ethanol-based; handle with care.
- Avoid ingestion and inhalation of vapors or dust.
- Follow OSHA, EPA, and laboratory safety regulations.
- Material Safety Data Sheet (MSDS) available upon request.
Additional information
| Size | 60mL Dropper Bottle (2 Fl Oz), 500mL (16 Fl Oz) w/ Dropper Bottle, 1000mL w/ Dropper Bottle (32 FL Oz) |
|---|
Related products
-

Copper Sulfate Pentahydrate | Food/Feed Grade
$19.99 – $49.99 Select options This product has multiple variants. The options may be chosen on the product page -

Ferrofluid Celestial Blue Elegance Display (New & Improved Blue Colored 2oz)
$19.99 Read more -

Copper (II) Carbonate – High Purity Solid
$14.99 – $45.00 Select options This product has multiple variants. The options may be chosen on the product page -

Anisyl Alcohol (4-Methoxybenzyl Alcohol)
$9.99 – $29.99 Select options This product has multiple variants. The options may be chosen on the product page
SKU: N/A
Categories: Dyes (2), Ph Indicators (5), Uncategorized
Tags: accurate pH indicator, Acid Testing Solution, acid-base indicator, Acid-Base Reactions, Acid-Base Titration, Analytical Reagents, Base Testing Solution, buy phenolphthalein online, buy phenolphthalein solution, Chemical Indicator, chemical indicator phenolphthalein, Chemistry Supplies, Educational Lab Equipment, Environmental Testing, Forensic Supplies, Industrial pH Indicator, Kastle-Meyer Test, lab-grade acid-base indicator, lab-grade phenolphthalein, Laboratory Chemicals, Laboratory Grade Indicator, pH Indicator, pH indicator phenolphthalein, pH Testing Kit, pH Testing Solution, pH Transition Range, Phenolphthalein, Phenolphthalein 1%, phenolphthalein acid-base reaction, phenolphthalein analytical grade, phenolphthalein aqueous solution, phenolphthalein bulk supplier, phenolphthalein bulk supply, phenolphthalein chemical properties, phenolphthalein color change, phenolphthalein distributor, phenolphthalein dropper bottle, phenolphthalein easy application, phenolphthalein environmental testing, phenolphthalein ethanol-based solution, phenolphthalein FDA compliant, phenolphthalein for academic research, phenolphthalein for acid detection, phenolphthalein for acid neutralization, phenolphthalein for aerospace research, phenolphthalein for agricultural applications, phenolphthalein for AI-driven chemistry, phenolphthalein for alkalinity measurement, phenolphthalein for alkalinity testing, phenolphthalein for alternative chemistry, phenolphthalein for analytical chemistry, phenolphthalein for analytical instruments, phenolphthalein for atmospheric studies, phenolphthalein for automation systems, phenolphthalein for bio-based analysis, phenolphthalein for biochemistry, phenolphthalein for biological research, phenolphthalein for biomolecular chemistry, phenolphthalein for biotechnology, phenolphthalein for blockchain traceability, phenolphthalein for carbon capture research, phenolphthalein for chemical analysis, phenolphthalein for chemical experiments, phenolphthalein for chemical laboratories, phenolphthalein for chemical manufacturing, phenolphthalein for chemical reaction tracking, phenolphthalein for chemical spill kits, phenolphthalein for chemical synthesis, phenolphthalein for chemical testing, phenolphthalein for chemistry, phenolphthalein for chemistry experiments, phenolphthalein for chemistry labs, phenolphthalein for CO2 absorption testing, phenolphthalein for commercial laboratories, phenolphthalein for controlled experiments, phenolphthalein for corrosion testing, phenolphthalein for cosmetic formulations, phenolphthalein for cosmetics industry, phenolphthalein for defense industry, phenolphthalein for detergent formulation, phenolphthalein for detergent manufacturing, phenolphthalein for drinkable water analysis, phenolphthalein for eco-friendly testing, phenolphthalein for educational purposes, phenolphthalein for educational use, phenolphthalein for emergency response, phenolphthalein for environmental monitoring, phenolphthalein for environmental protection agencies, phenolphthalein for environmental safety, phenolphthalein for environmental science, phenolphthalein for environmental testing, phenolphthalein for enzyme studies, phenolphthalein for fluid chemistry, phenolphthalein for food industry testing, phenolphthalein for food processing, phenolphthalein for food safety testing, phenolphthalein for forensic analysis, phenolphthalein for forensic testing, phenolphthalein for gastric acid testing, phenolphthalein for genetic research, phenolphthalein for global distribution, phenolphthalein for government testing, phenolphthalein for green chemistry, phenolphthalein for hazardous material handling, phenolphthalein for high school chemistry, phenolphthalein for hydrogen fuel cells, phenolphthalein for hydroponics, phenolphthalein for industrial applications, phenolphthalein for industrial automation, phenolphthalein for industrial compliance, phenolphthalein for industrial quality control, phenolphthalein for industrial R&D, phenolphthalein for industrial testing, phenolphthalein for industrial titration, phenolphthalein for industrial wastewater, phenolphthalein for ink formulations, phenolphthalein for innovative research, phenolphthalein for interactive learning, phenolphthalein for IoT applications, phenolphthalein for laboratories, phenolphthalein for laboratory analysis, phenolphthalein for laboratory safety, phenolphthalein for laboratory use, phenolphthalein for medical diagnostics, phenolphthalein for medical testing, phenolphthalein for microbiology, phenolphthalein for microfluidics, phenolphthalein for military applications, phenolphthalein for molecular diagnostics, phenolphthalein for nanotechnology, phenolphthalein for non-hazardous applications, phenolphthalein for non-toxic formulations, phenolphthalein for organic chemistry, phenolphthalein for personal care formulations, phenolphthalein for pesticide formulation, phenolphthalein for pH measurement, phenolphthalein for pH monitoring, phenolphthalein for pH testing, phenolphthalein for pharmaceutical industry, phenolphthalein for pharmaceutical research, phenolphthalein for pollution monitoring, phenolphthalein for precision measurement, phenolphthalein for predictive maintenance, phenolphthalein for process control, phenolphthalein for protein analysis, phenolphthalein for quality assurance, phenolphthalein for quality control, phenolphthalein for rapid response testing, phenolphthalein for reagent kits, phenolphthalein for renewable applications, phenolphthalein for research, phenolphthalein for research facilities, phenolphthalein for school experiments, phenolphthalein for scientific experiments, phenolphthalein for scientific research, phenolphthalein for smart chemistry, phenolphthalein for smart laboratories, phenolphthalein for soap making, phenolphthalein for soil testing, phenolphthalein for space exploration, phenolphthalein for spill containment, phenolphthalein for STEM education, phenolphthalein for strong base identification, phenolphthalein for supply chain optimization, phenolphthalein for sustainable chemistry, phenolphthalein for textile processing, phenolphthalein for titration, phenolphthalein for titration kits, phenolphthalein for titrations, phenolphthalein for universities, phenolphthalein for university chemistry, phenolphthalein for university labs, phenolphthalein for urine analysis, phenolphthalein for wastewater analysis, phenolphthalein for wastewater management, phenolphthalein for wastewater treatment, phenolphthalein for water quality analysis, phenolphthalein for water testing, phenolphthalein high purity, phenolphthalein in ethanol, phenolphthalein in water, Phenolphthalein Indicator, phenolphthalein indicator solution, phenolphthalein industrial grade, phenolphthalein ISO certified, phenolphthalein lab reagent, phenolphthalein lab supplies, phenolphthalein laboratory grade, phenolphthalein laboratory reagent, phenolphthalein manufacturer, phenolphthalein MSDS, phenolphthalein non-toxic indicator, phenolphthalein online supplier, phenolphthalein pH test, phenolphthalein pH testing, phenolphthalein quality assured, phenolphthalein REACH compliant, phenolphthalein reagent, phenolphthalein reagent grade, phenolphthalein regulatory compliance, phenolphthalein RoHS compliant, phenolphthalein safety data sheet, phenolphthalein shipping regulations, phenolphthalein soil testing, Phenolphthalein Solution, phenolphthalein storage guidelines, phenolphthalein titration reagent, phenolphthalein wholesale, phenolphthalein with dropper bottle, Science Experiment Supplies, titration indicator, Titration Supplies, Water Testing Kit