Zinc Chloride Purified – High-Quality Chemical Compound
$12.00 – $59.99
General Information
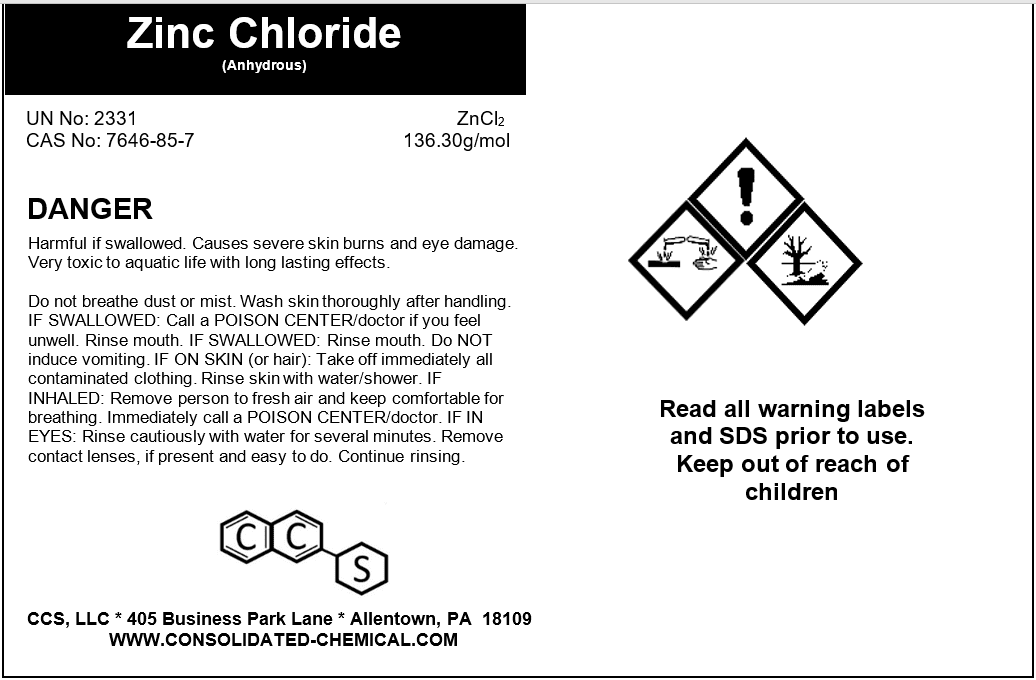
- Chemical Name: Zinc Chloride
- Molecular Formula: ZnCl₂
- CAS Number: 7646-85-7
- EC Number: 231-592-0
- Appearance: White, crystalline powder
- Odor: Odorless
Chemical Properties
- Purity: ≥99%
- Molecular Weight: 136.30 g/mol
- Solubility: Highly soluble in water, alcohol, and ether
- Melting Point: 290°C (554°F)
- Boiling Point: Sublimes at 732°C (1,350°F)
- Density: 2.91 g/cm³ at 25°C
Physical Properties
- pH (in Aqueous Solution): ~4–5 (for a 5% solution)
- Hygroscopic Nature: Absorbs moisture from the air; deliquescent
- Form: Fine powder or granules
Description
Unlock the potential of a versatile chemical compound with our high-purity Zinc Chloride. Ideal for industrial, laboratory, and DIY applications, this premium-grade compound ensures reliability and precision in every use. Whether you’re conducting scientific research, crafting homemade solutions, or working on specialized projects, our Zinc Chloride offers exceptional performance.
Applications of Zinc Chloride Purified
Industrial Applications
- Galvanizing:Acts as a flux in the hot-dip galvanizing process, cleaning the metal surface and promoting adhesion of zinc coatings.
- Textile Processing:Used in dyeing and textile finishing processes for its ability to modify fabric properties.
- Soldering Flux:Commonly used as a flux to clean metal surfaces and improve solder flow and adhesion.
- Rubber Manufacturing:Plays a role in vulcanization processes to improve rubber elasticity and durability.
- Petroleum Industry:Used in oil refining as a catalyst and dehydrating agent.
Laboratory Applications
- Organic Synthesis:Serves as a Lewis acid in various chemical reactions, including Friedel-Crafts alkylation and acylation.
- Analytical Chemistry:Acts as a reagent for qualitative and quantitative analysis of specific compounds.
- Preparation of Specialty Chemicals:Used in the synthesis of compounds like zinc-based catalysts and other derivatives.
Water Treatment
- Corrosion Inhibition:Used in water treatment plants to reduce pipe corrosion and prolong system lifespan.
- Disinfectant:Its antimicrobial properties make it suitable for specific water purification applications.
Construction Industry
- Wood Preservation:Used in treating wood to protect against rot, insects, and fungal decay.
- Cement Accelerant:Improves the setting time and strength of concrete mixes.
Personal Care & Cosmetics
- Antiperspirant Formulations:Used as an ingredient in deodorants and antiperspirants for its astringent properties.
- Hair Care:Sometimes included in hair dye products for its role in color setting.
Electronics and Batteries
- Battery Production:Serves as an electrolyte component in dry cell batteries and other energy storage systems.
- Printed Circuit Boards (PCBs):Utilized in etching solutions for PCB manufacturing.
DIY and Hobbyist Projects
- Metal Cleaning:Used as a cleaning agent for metals before welding or soldering.
- Chemical Experiments:A popular reagent for home-based chemistry experiments due to its reactivity and accessibility.
Agriculture
- Fertilizer Additive:Provides zinc, an essential micronutrient for plant growth, in certain agricultural applications.
Storage:
- Storage Conditions:
- Keep Zinc Chloride in a cool, dry, and well-ventilated area to avoid moisture absorption.
- Store in a tightly sealed container to maintain purity and prevent contamination.
- Avoid exposure to direct sunlight or extreme temperatures, as these conditions may degrade the product.
- Segregation:
- Store away from incompatible substances such as strong bases, water, and oxidizing agents.
- Use a dedicated storage area for chemicals to minimize the risk of cross-contamination.
- Packaging Integrity:
- Check the container regularly for signs of leakage or damage. Replace if necessary.
- Labeling:
- Ensure the container is clearly labeled to avoid accidental misuse.
Handling:
- Personal Protective Equipment (PPE):
- Wear protective gloves (rubber or nitrile) to avoid skin contact.
- Use safety goggles to protect your eyes.
- Wear a lab coat or protective clothing to prevent skin exposure.
- If handling in powdered form, use a dust mask or respirator to prevent inhalation of particles.
- Work Environment:
- Always handle in a well-ventilated area or under a fume hood to avoid inhaling fumes or dust.
- Keep the workspace clean and free from clutter.
- Precautionary Measures:
- Avoid inhalation, ingestion, or prolonged skin contact.
- Do not mix Zinc Chloride with incompatible substances such as water, as it may produce heat or fumes.
- Handle with care to prevent spills. If spills occur, clean them immediately using appropriate absorbent materials.
- Emergency Preparedness:
- Ensure easy access to an eye wash station and safety shower in case of accidental exposure.
- Keep an appropriate spill kit nearby for safe cleanup.
Disposal:
- Dispose of Zinc Chloride and its containers in accordance with local, state, and federal regulations.
- Do not discharge into sewers or waterways.
Additional information
| Size | 50 Grams, 250 Grams, 500 Grams, 1000 Grams |
|---|
Related products
-

X-100 Nonionic Surfactant – High Performance
$15.99 – $99.99 Select options This product has multiple variants. The options may be chosen on the product page -

Ferrofluid Magnetic Liquid
$12.00 – $260.00 Select options This product has multiple variants. The options may be chosen on the product page -

Ammonium Hydroxide 29% – Premium Aqueous Solution
$14.99 – $29.99 Select options This product has multiple variants. The options may be chosen on the product page -

Potassium Carbonate – Food Grade (E501)
$24.99 – $39.99 Select options This product has multiple variants. The options may be chosen on the product page
SKU: N/A
Categories: Fertilizer, Fertizlizer Additive, Industrial Chemical, Water Treatment
Tags: 7646-85-7, Affordable Zinc Chloride, Analytical Reagent Zinc Chloride, Bulk Zinc Chloride Supplier, buy zinc chloride, Buy Zinc Chloride Online, CAS 7646-85-7, DIY Zinc Chloride Uses, Eco-Friendly Zinc Chloride Packaging, Galvanizing Zinc Chloride, High-Purity Zinc Chloride, Lab Use Zinc Chloride, Laboratory-Grade Zinc Chloride, Long Shelf-Life Zinc Chloride, Premium Zinc Chloride Compound, purified zinc chloride, Resealable Zinc Chloride Container, Rubber Manufacturing Zinc Chloride, Safe Zinc Chloride Packaging, Small Packaged Zinc Chloride, Soldering Flux Zinc Chloride, Textile Processing Zinc Chloride, Wood Preservative Zinc Chloride, zinc chloride 99.9%, zinc chloride 99%, zinc chloride ACS grade, zinc chloride advanced formulations, zinc chloride alloying agent, zinc chloride anhydrous, zinc chloride applications, zinc chloride as stabilizer, zinc chloride best formulation practices, zinc chloride best supplier, zinc chloride biodegradable, zinc chloride boiling point, zinc chloride bulk pricing, zinc chloride bulk supplier, zinc chloride CAS 7646-85-7, zinc chloride catalyst applications, Zinc Chloride Chemical, zinc chloride chemical safety, zinc chloride chemical structure, zinc chloride commercial applications, zinc chloride competitive analysis, zinc chloride conductivity, zinc chloride corrosion inhibitor, zinc chloride cost, zinc chloride cost-effective solutions, zinc chloride critical raw material, zinc chloride crystalline, zinc chloride decomposition, zinc chloride deliquescent, zinc chloride demand forecast, zinc chloride density, zinc chloride distributor, zinc chloride DOT regulations, zinc chloride eco-friendly, zinc chloride electrolyte, zinc chloride emergency response, zinc chloride emerging markets, zinc chloride energy-efficient formulations, zinc chloride environmental impact, zinc chloride EPA regulations, zinc chloride EU regulations, zinc chloride exposure risks, zinc chloride FDA approved, zinc chloride flexible applications, zinc chloride food grade, zinc chloride food processing, Zinc Chloride for Chemical Experiments, Zinc Chloride for Construction, Zinc Chloride for Hobby Projects, Zinc Chloride for Home Chemistry, Zinc Chloride for Industrial Use, Zinc Chloride for Metal Cleaning, Zinc Chloride for Metal Crafts, Zinc Chloride for Organic Synthesis, Zinc Chloride for Sale, Zinc Chloride for Water Treatment, zinc chloride GHS classification, zinc chloride global market, zinc chloride green chemistry, zinc chloride handling precautions, zinc chloride hazard classification, zinc chloride high purity, Zinc Chloride High Quality, zinc chloride high-performance formulations, zinc chloride hydrate, zinc chloride hydrolysis, zinc chloride hygroscopic, zinc chloride import/export, zinc chloride in adhesives, zinc chloride in advanced materials, zinc chloride in agrochemicals, zinc chloride in air fresheners, zinc chloride in alternative chemistry, zinc chloride in alternative fuels, zinc chloride in analytical chemistry, zinc chloride in animal feed, zinc chloride in anti-corrosion coatings, Zinc Chloride in Batteries, zinc chloride in biocides, zinc chloride in biotechnology, zinc chloride in catalyst, zinc chloride in chemical processing, zinc chloride in chemical synthesis, zinc chloride in chromatography, zinc chloride in clean technology, zinc chloride in coatings, zinc chloride in controlled reactions, zinc chloride in cosmetics, zinc chloride in degreasers, zinc chloride in deodorants, zinc chloride in detergents, zinc chloride in dietary supplements, zinc chloride in disinfectants, zinc chloride in drug formulations, zinc chloride in dyes, zinc chloride in electronics, zinc chloride in electroplating, zinc chloride in emerging technologies, zinc chloride in energy storage, zinc chloride in environmental applications, zinc chloride in fertilizers, zinc chloride in fine chemicals, zinc chloride in flame retardants, zinc chloride in flux, zinc chloride in food additives, zinc chloride in food preservation, zinc chloride in forensic science, zinc chloride in fuel cells, zinc chloride in galvanizing, zinc chloride in global trade, zinc chloride in haircare, zinc chloride in herbicides, zinc chloride in high-performance coatings, zinc chloride in high-tech manufacturing, zinc chloride in household cleaners, zinc chloride in hydrogen production, zinc chloride in industrial cleaning, zinc chloride in laboratory applications, zinc chloride in lubricants, zinc chloride in medical research, zinc chloride in metal alloys, zinc chloride in metal finishing, zinc chloride in nanotechnology, zinc chloride in nutritional formulations, zinc chloride in paints, zinc chloride in personal care, zinc chloride in pesticides, zinc chloride in petrochemicals, zinc chloride in pharmaceuticals, zinc chloride in pigments, zinc chloride in plasticizers, zinc chloride in pollution control, zinc chloride in polymer chemistry, zinc chloride in renewable energy, zinc chloride in rubber processing, zinc chloride in rust prevention, zinc chloride in sanitizers, zinc chloride in semiconductor industry, zinc chloride in sensors, zinc chloride in skincare, zinc chloride in soaps, zinc chloride in soil treatment, zinc chloride in soldering, zinc chloride in specialty chemicals, zinc chloride in spectroscopy, zinc chloride in surface treatment, zinc chloride in sustainable production, zinc chloride in textile treatment, zinc chloride in textiles, zinc chloride in veterinary medicine, zinc chloride in wastewater treatment, zinc chloride in water purification, zinc chloride in water treatment, zinc chloride in welding, zinc chloride industrial applications, zinc chloride industrial grade, zinc chloride industrial R&D, zinc chloride industrial safety, zinc chloride industry trends, zinc chloride industry-leading product, zinc chloride innovative applications, zinc chloride innovative solutions, zinc chloride laboratory grade, zinc chloride manufacturer, zinc chloride melting point, zinc chloride molecular formula, zinc chloride molecular weight, zinc chloride MSDS, zinc chloride next-generation applications, zinc chloride online purchase, zinc chloride oxidation, zinc chloride passivation, zinc chloride pharmaceutical grade, zinc chloride polarity, zinc chloride powder, zinc chloride precision manufacturing, zinc chloride premium quality, zinc chloride price, Zinc Chloride Purified, zinc chloride REACH certified, zinc chloride reactivity, zinc chloride reagent grade, zinc chloride refractive index, zinc chloride regulatory compliance, zinc chloride research and development, zinc chloride safe shipping, zinc chloride safety data sheet, zinc chloride safety guidelines, zinc chloride scientific studies, zinc chloride SDS, zinc chloride solubility, zinc chloride solution, zinc chloride spill management, zinc chloride stability, zinc chloride storage conditions, Zinc Chloride Storage Instructions, zinc chloride strategic sourcing, zinc chloride supply chain management, zinc chloride supply chain optimization, zinc chloride sustainable alternatives, zinc chloride sustainable chemistry, zinc chloride toxicity, zinc chloride transport regulations, zinc chloride US regulations, zinc chloride vapor pressure, zinc chloride verified distributor, zinc chloride verified supplier, zinc chloride waste disposal, zinc chloride wholesale