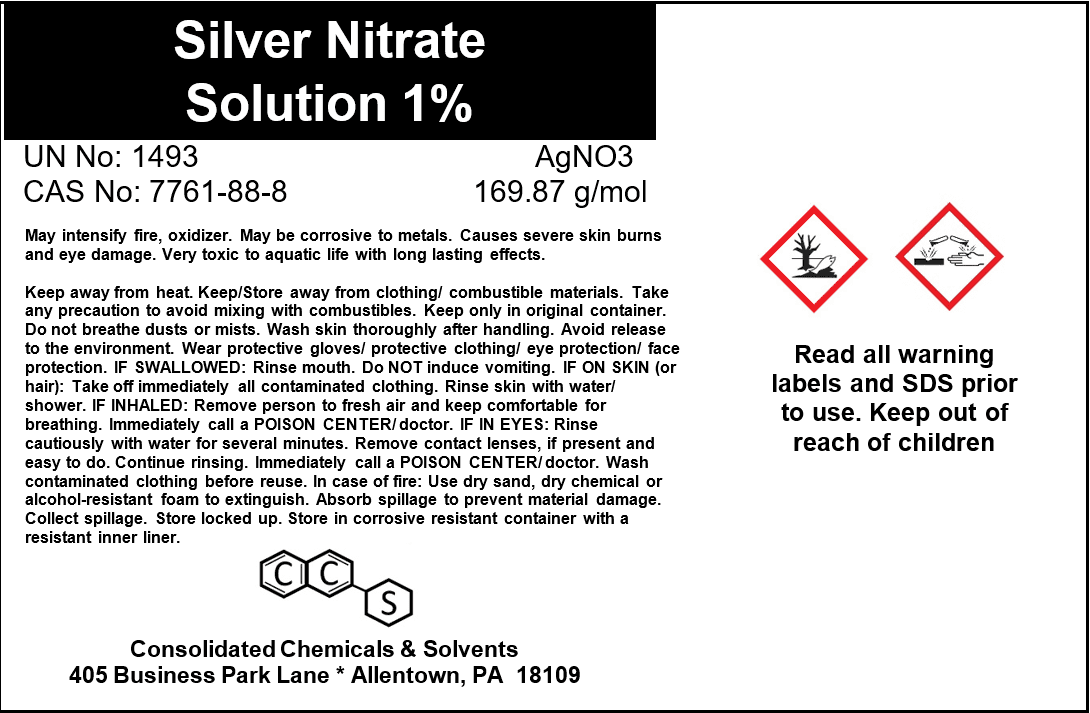
Silver Nitrate Solution 1% | Reagent Grade
$10.50
General Properties
- Chemical Name: Silver Nitrate Solution
- Concentration: 1% w/v (1 g of Silver Nitrate per 100 mL of solution)
- CAS Number: 7761-88-8 (Silver Nitrate)
- Chemical Formula: AgNO₃
- Appearance: Clear, colorless solution
- Odor: Odorless
- Solvent: Distilled or deionized water
- Molecular Weight: 169.87 g/mol (Silver Nitrate)
- pH: Slightly acidic (~5.0–6.5)
- Density (@ 25°C): ~1.0 g/cm³
Purity and Composition
- Impurities: Negligible levels of heavy metals (below detection limits).
- Water Quality: Distilled or deionized, free from impurities.
Physical and Chemical Properties
- Boiling Point: ~100°C (water-based solution)
- Freezing Point: ~0°C (water-based solution)
- Solubility: Completely soluble in water
- Stability: Stable under recommended storage conditions. Light-sensitive; may degrade upon prolonged exposure to light.
Documents:
Description
Silver Nitrate Solution 1% is a high-purity, laboratory-grade chemical designed for a variety of research, educational, and laboratory uses. Known for its excellent chemical properties, this solution is suitable for analytical testing, chemical synthesis, and experiments requiring precise concentrations. It is packaged in a secure, easy-to-handle container to ensure safe usage and storage.
Applications of Silver Nitrate Solution 1%
Laboratory & Analytical Applications
- Precipitation Reagent: Used for chloride ion detection in analytical chemistry.
- Volumetric Analysis (Titration): Employed in argentometric titrations.
- Microbiological Staining: Utilized in Gram staining and bacterial identification.
- pH & Ion Testing: Reacts with halide ions (Cl⁻, Br⁻, I⁻) to form precipitates.
Medical & Pharmaceutical Applications
- Antiseptic & Disinfectant: Historically used as a mild antimicrobial agent.
- Wound Treatment: Used in cauterization and wart removal (under medical supervision).
- Eye Drops: In the past, used in newborn eye prophylaxis against infections.
Industrial & Chemical Applications
- Silver Plating & Metallurgy: Used in electroplating and metal refining.
- Photography & Film Processing: Involved in photo-sensitive material production.
- Glass & Mirror Manufacturing: Enhances reflectivity in coated surfaces.
- Dye & Pigment Industry: Used in specialized textile staining.
Environmental & Water Testing
- Chloride Content Analysis: Determines chloride levels in drinking and wastewater.
- Heavy Metal Precipitation: Aids in environmental and industrial water treatment.
Storage
- Store in a cool, dry, and dark environment.
- Keep in light-resistant (amber) containers to prevent decomposition.
- Avoid contact with organic materials and strong reducing agents.
Handling
- Use protective gloves, safety goggles, and lab coats when handling.
- Avoid contact with skin and eyes; can cause staining and irritation.
- Use in well-ventilated areas to prevent buildup of vapors.
Safety
- Oxidizing agent; may react with combustible substances.
- Avoid inhalation and ingestion; can be toxic in high concentrations.
- Follow OSHA, EPA, and industrial safety regulations.
- Material Safety Data Sheet (MSDS) available upon request.
Additional information
| Size | 30mL (1 Fl Oz) |
|---|
Related products
-
Methylene Chloride (Dichloromethane)
$28.00 – $135.00 Select options This product has multiple variants. The options may be chosen on the product page -

Ferrofluid Celestial Blue Elegance Display (New & Improved Blue Colored 2oz)
$19.99 Read more -

Copper (II) Carbonate – High Purity Solid
$14.99 – $45.00 Select options This product has multiple variants. The options may be chosen on the product page -
Methyl Eugenol High Purity Aroma Compound
$11.00 – $39.99 Select options This product has multiple variants. The options may be chosen on the product page
SKU: N/A
Categories: Ph Indicators (5), Research, Uncategorized
Tags: 1% silver nitrate for titration, 1% silver nitrate solution, 1% silver nitrate solution bulk, AgNO₃ solution, analytical chemistry reagent, analytical reagent silver nitrate, antimicrobial silver nitrate, antimicrobial silver nitrate solution, argentometric titration reagent, argentometric titration solution, buy silver nitrate solution, chemical reagent silver nitrate, chloride detection solution, High-Purity Silver Nitrate, high-purity silver nitrate solution, laboratory chemical silver nitrate, Laboratory Grade Silver Nitrate, laboratory reagent silver nitrate, reagent-grade 1% silver nitrate, reagent-grade silver nitrate, reagent-grade silver nitrate for staining, reagent-grade silver nitrate solution, silver nitrate 1% solution bulk, silver nitrate analytical reagent, silver nitrate antimicrobial properties, silver nitrate antiseptic solution, silver nitrate aqueous solution, silver nitrate bulk supplier, silver nitrate chemical properties, silver nitrate distributor, silver nitrate eye drops, silver nitrate for adhesive manufacturing, silver nitrate for advanced materials, silver nitrate for advanced propulsion systems, silver nitrate for aerospace coatings, silver nitrate for agriculture, silver nitrate for AI-powered analysis, silver nitrate for alternative energy, silver nitrate for analytical chemistry, silver nitrate for antimicrobial coatings, silver nitrate for antiseptic use, silver nitrate for artificial intelligence applications, silver nitrate for aviation technology, silver nitrate for bacterial infections, silver nitrate for batch testing, silver nitrate for beverage testing, silver nitrate for big data analytics in chemical production, silver nitrate for bioimaging, silver nitrate for biological staining, silver nitrate for biological trace analysis, silver nitrate for biomedical devices, silver nitrate for blockchain traceability, silver nitrate for burns treatment, silver nitrate for carbon footprint reduction, silver nitrate for cell culture, silver nitrate for chemical catalysis, silver nitrate for chemical hazard detection, silver nitrate for chemical synthesis, silver nitrate for chemistry experiments, silver nitrate for chloride detection, silver nitrate for circular economy, silver nitrate for clean energy research, silver nitrate for coatings, silver nitrate for coin production, silver nitrate for compliance testing, silver nitrate for connected devices, silver nitrate for controlled release formulations, silver nitrate for corrosion testing, silver nitrate for cybersecurity in chemical industry, silver nitrate for defense industry, silver nitrate for dental applications, silver nitrate for diagnostic assays, silver nitrate for dialysis treatments, silver nitrate for digital twin technology, silver nitrate for disinfectants, silver nitrate for DNA testing, silver nitrate for drug delivery systems, silver nitrate for drug formulation, silver nitrate for eco-friendly manufacturing, silver nitrate for education, silver nitrate for educational experiments, silver nitrate for electric vehicle battery development, silver nitrate for electrochemical analysis, silver nitrate for electronics industry, silver nitrate for electroplating, silver nitrate for emergency response, silver nitrate for energy storage, silver nitrate for environmental analysis, silver nitrate for environmental monitoring, silver nitrate for environmental protection, silver nitrate for enzyme activity assays, silver nitrate for explosives detection, silver nitrate for eye treatment, silver nitrate for FDA-approved applications, silver nitrate for fluorescence studies, silver nitrate for food contact materials, silver nitrate for food safety testing, silver nitrate for forensic fingerprinting, silver nitrate for forensic science, silver nitrate for forensic testing, silver nitrate for fuel cell development, silver nitrate for genetic research, silver nitrate for geological analysis, silver nitrate for glass manufacturing, silver nitrate for GMP-certified production, silver nitrate for green chemistry, silver nitrate for halide testing, silver nitrate for healthcare industry, silver nitrate for heavy metal detection, silver nitrate for histochemical staining, silver nitrate for histology, silver nitrate for hydrogen production, silver nitrate for immunology research, silver nitrate for industrial applications, silver nitrate for industrial automation, silver nitrate for industrial effluent treatment, silver nitrate for industrial hygiene, silver nitrate for industrial IoT, silver nitrate for industrial safety, silver nitrate for ink formulations, silver nitrate for jewelry making, silver nitrate for laboratory analysis, silver nitrate for laboratory use, silver nitrate for leather treatment, silver nitrate for LED manufacturing, silver nitrate for logistics tracking, silver nitrate for machine learning applications, silver nitrate for material science, silver nitrate for medical coatings, silver nitrate for medical diagnostics, silver nitrate for medical imaging, silver nitrate for metal surface treatment, silver nitrate for metallurgy, silver nitrate for metallurgy testing, silver nitrate for microbiology, silver nitrate for microscopy, silver nitrate for military applications, silver nitrate for mining industry, silver nitrate for nano silver synthesis, silver nitrate for nanomedicine, silver nitrate for nanotechnology, silver nitrate for neuroscience, silver nitrate for non-destructive testing, silver nitrate for ophthalmology, silver nitrate for organ preservation, silver nitrate for packaging industry, silver nitrate for paint industry, silver nitrate for pathology, silver nitrate for petrochemical industry, silver nitrate for pharmaceutical excipients, silver nitrate for pharmaceutical quality control, silver nitrate for pharmaceutical stability testing, silver nitrate for pharmaceutical synthesis, silver nitrate for pharmaceuticals, silver nitrate for photography, silver nitrate for pigment production, silver nitrate for plastic manufacturing, silver nitrate for polymer processing, silver nitrate for precision engineering, silver nitrate for predictive maintenance, silver nitrate for protein analysis, silver nitrate for quality control, silver nitrate for rare earth element extraction, silver nitrate for raw material verification, silver nitrate for real-time monitoring, silver nitrate for regenerative medicine, silver nitrate for regulatory compliance, silver nitrate for regulatory reporting, silver nitrate for research applications, silver nitrate for resource recovery, silver nitrate for robotics, silver nitrate for rocket fuel additives, silver nitrate for scientific innovation, silver nitrate for scientific research, silver nitrate for semiconductor etching, silver nitrate for semiconductor fabrication, silver nitrate for sensor applications, silver nitrate for silver ion reactions, silver nitrate for silver plating, silver nitrate for smart manufacturing, silver nitrate for soil testing, silver nitrate for solar panels, silver nitrate for space exploration, silver nitrate for space research, silver nitrate for spectroscopy, silver nitrate for spill containment, silver nitrate for staining, silver nitrate for sterilization processes, silver nitrate for structural analysis, silver nitrate for supply chain optimization, silver nitrate for sustainability initiatives, silver nitrate for tattoo removal, silver nitrate for testing, silver nitrate for textile dyeing, silver nitrate for textile industry, silver nitrate for tissue engineering, silver nitrate for tissue staining, silver nitrate for titration, silver nitrate for topical use, silver nitrate for toxicology studies, silver nitrate for vaccine production, silver nitrate for veterinary medicine, silver nitrate for waste management solutions, silver nitrate for waste recycling, silver nitrate for wastewater treatment, silver nitrate for water purification, silver nitrate for water testing, silver nitrate for wound care, silver nitrate in aqueous medium, silver nitrate ISO certified, silver nitrate light-sensitive, silver nitrate manufacturer, silver nitrate medical applications, silver nitrate MSDS, silver nitrate online supplier, silver nitrate oxidation reactions, silver nitrate oxidizing agent, silver nitrate REACH compliant, silver nitrate reagent grade, silver nitrate RoHS compliant, silver nitrate safety data sheet, silver nitrate shipping regulations, Silver Nitrate Solution 1%, silver nitrate solution for research, silver nitrate storage guidelines, silver nitrate water testing, silver nitrate water testing solution, silver nitrate wound cauterization