Triethylamine – Premium Organic Solvent – Laboratory Reagent
$19.99 – $39.99
Chemical Information:
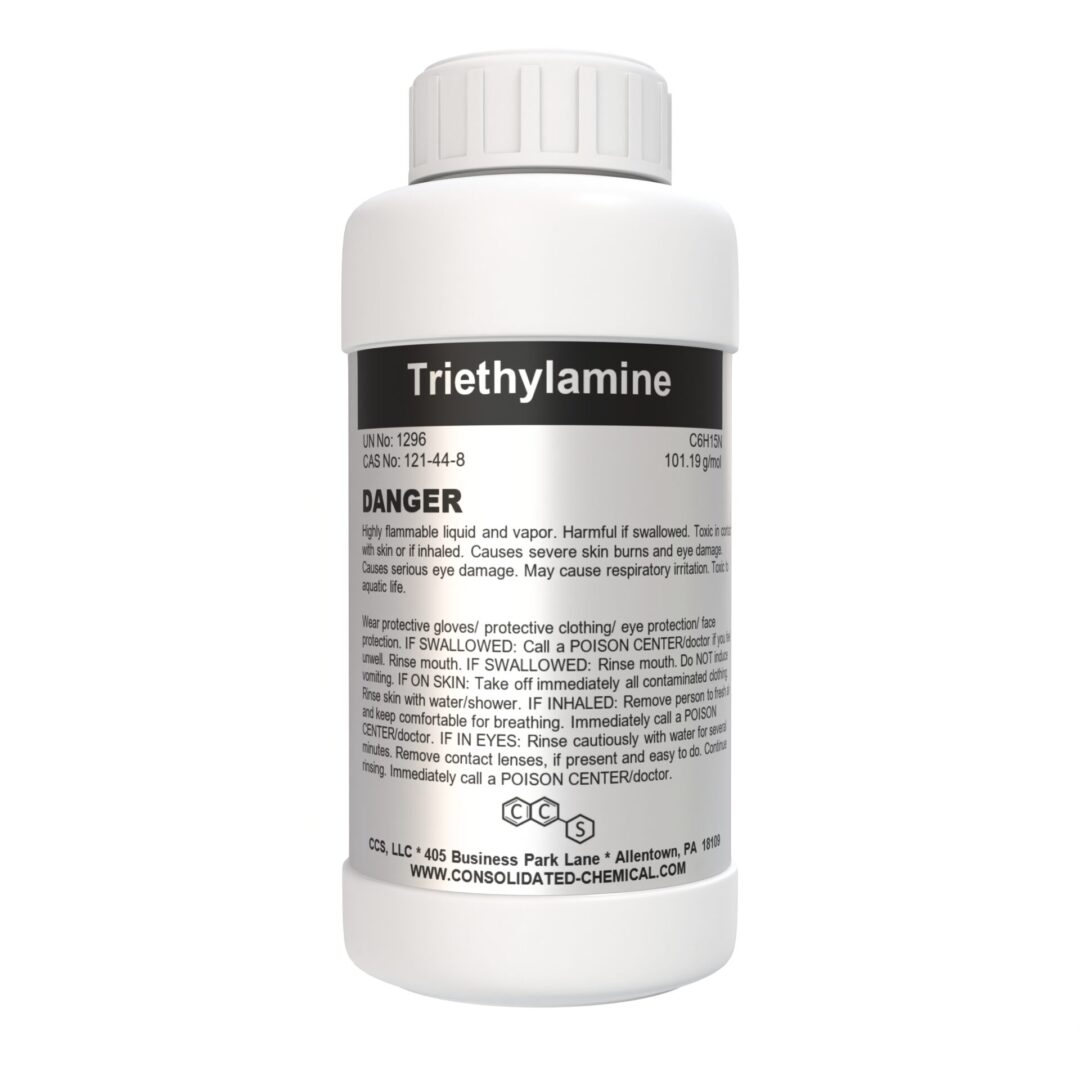
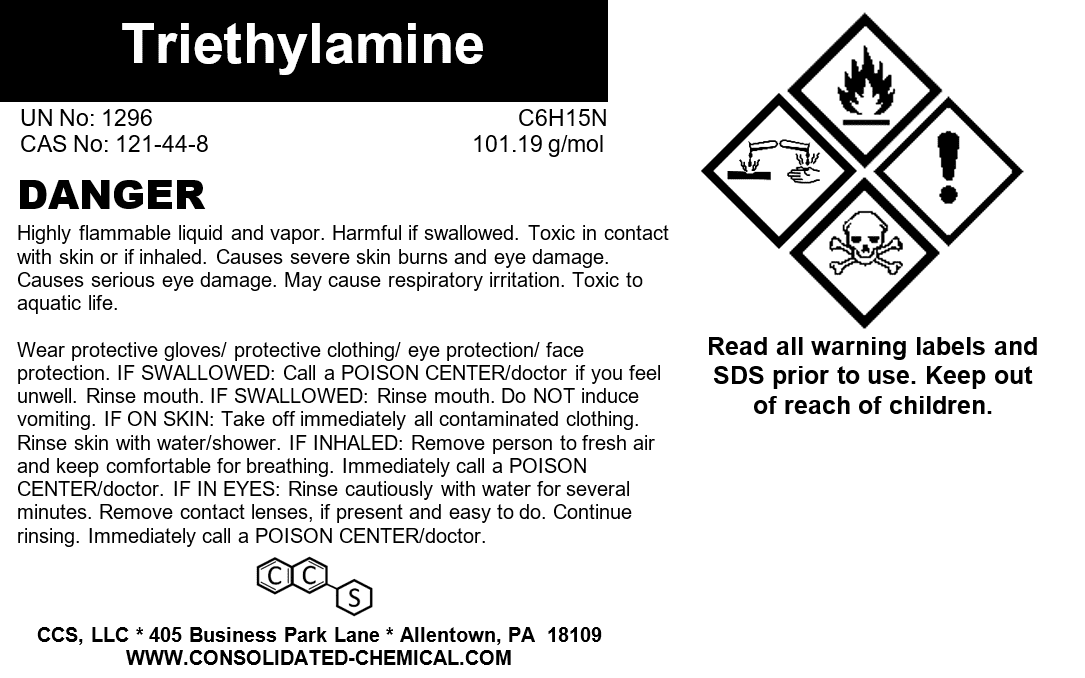
- Chemical Name: Triethylamine
- Molecular Formula: C6H15N
- Molecular Weight: 101.19 g/mol
- CAS Number: 121-44-8
Physical Properties:
- Appearance: Clear, colorless liquid
- Odor: Strong, ammonia-like odor
- Boiling Point: ~89°C (192°F)
- Melting Point: ~-114°C (-173°F)
- Density: ~0.726 g/cm³ at 20°C (68°F)
- Refractive Index: 1.400–1.405 at 20°C
- Vapor Pressure: ~57 mmHg at 20°C (volatile)
- Solubility:
- Miscible with water, ethanol, acetone, and most organic solvents
Chemical Properties:
- pH: Strongly basic (aqueous solutions)
- Flash Point: -11°C (12°F), closed cup (highly flammable)
- Autoignition Temperature: ~215°C (419°F)
- Explosive Limits: 1.2%–8.0% (volume in air)
Documents :
Description
Triethylamine is a high-purity organic solvent and reagent designed for laboratory and industrial applications. Its versatility and efficiency make it a critical component in a wide range of chemical synthesis and manufacturing processes. With exceptional purity and stability, our Triethylamine ensures reliable results in both research and production settings.
Applications of Triethylamine
Chemical Synthesis
- Acid Neutralization: Acts as a strong organic base in chemical reactions.
- Catalyst: Frequently used in esterification, acylation, and transesterification reactions.
- Intermediate: A critical reagent for synthesizing esters, amides, and other nitrogen-containing compounds.
Pharmaceutical Industry
- Active Pharmaceutical Ingredients (API): Key role in synthesizing drugs and intermediates.
- Quaternary Ammonium Salts: Used to produce these compounds, which are essential in medicinal chemistry.
- Buffer Agent: Maintains pH stability during pharmaceutical processes.
Agrochemical Industry
- Pesticides: Intermediate in manufacturing herbicides, fungicides, and insecticides.
- Fertilizer Production: Contributes to nitrogen-based fertilizer synthesis.
Polymer Industry
- Polymerization Catalyst: Aids in the production of polycarbonates and epoxies.
- Resin Curing: Acts as a hardener or curing agent in resin formulations.
Laboratory Applications
- Chromatography: Additive for mobile phases in high-performance liquid chromatography (HPLC).
- Buffer Solutions: Ensures stable pH levels for experiments and reactions.
- Neutralizing Agent: Neutralizes acidic residues during synthesis.
Coatings and Paints
- Corrosion Inhibitor: Enhances the durability of anti-corrosive coatings.
- Stabilizer: Improves the stability of paint and coating formulations.
Textile Industry
- Dye Fixation: Facilitates the bonding of dyes to textiles.
- Processing Aid: Adjusts pH during fabric treatment and finishing processes.
Fuel and Energy
- Fuel Additive: Stabilizes fuel mixtures for better combustion.
- Battery Manufacturing: Used in specific battery electrolyte formulations.
Rubber and Plastics
- Elastomer Production: Acts as a vulcanization catalyst for rubber products.
- Plastic Stabilizer: Enhances the physical properties of certain plastics.
Perfumes and Flavors
- Fragrance Synthesis: Assists in producing aromatic compounds for perfumes.
- Flavor Compounds: Intermediate in creating food-safe flavors.
Environmental Applications
- Air Scrubbing: Neutralizes acidic gases in industrial air treatment systems.
- Wastewater Treatment: Neutralizes and treats acidic waste streams.
Food and Beverage
- Processing Aid: Used sparingly in pH adjustments and specific food-grade formulations.
Education
- Research and Teaching: Used as a reagent in academic laboratories for teaching organic chemistry.
Storage:
- Temperature:
- Store in a cool, dry, and well-ventilated area, ideally below 25°C (77°F).
- Protect from direct sunlight and sources of heat to prevent vapor buildup.
- Containers:
- Store in tightly sealed, corrosion-resistant containers made of stainless steel or specific plastics compatible with amines.
- Avoid using containers made from materials that can react with amines.
- Environment:
- Keep away from oxidizing agents, acids, and sources of ignition.
- Store in a designated flammable liquid storage area.
- Ventilation:
- Ensure adequate ventilation to prevent accumulation of vapors, which can be flammable and harmful if inhaled.
- Secondary Containment:
- Use spill containment measures such as trays or dikes to prevent accidental release.
Handling:
- Personal Protective Equipment (PPE):
- Eye Protection: Wear chemical splash goggles or a face shield.
- Skin Protection: Use chemical-resistant gloves (e.g., nitrile or neoprene) and protective clothing.
- Respiratory Protection: Use a respirator with filters for organic vapors if working in areas with insufficient ventilation.
- Ventilation:
- Handle in a fume hood or a well-ventilated area to minimize exposure to vapors.
- Safe Practices:
- Avoid inhalation, ingestion, and skin or eye contact.
- Ground and bond containers during transfer to prevent static discharge.
- Do not eat, drink, or smoke while handling the chemical.
- Mixing:
- Add Triethylamine carefully when mixing with other chemicals, as it can react violently with oxidizing agents or acids.
Spill and Leak Management:
- Small Spills:
- Absorb with an inert material such as sand or vermiculite.
- Place the waste in a sealed, labeled container for proper disposal.
- Large Spills:
- Evacuate the area and restrict access.
- Use appropriate PPE and contain the spill to prevent environmental contamination.
- Ensure ventilation and eliminate all ignition sources.
Disposal:
- Dispose of Triethylamine and any contaminated materials in compliance with local, state, and federal regulations.
- Do not dispose of it down drains or into the environment.
Additional information
| Size | 100mL (3.3 Fl Oz), 250mL (8 Fl Oz), 500mL (16 Fl Oz), 1000mL (32 Fl Oz) |
|---|
Be the first to review “Triethylamine – Premium Organic Solvent – Laboratory Reagent” Cancel reply
Related products
-

Geraniol | High-Purity Fragrance/Aroma Compound
$9.99 – $800.00 Select options This product has multiple variants. The options may be chosen on the product page -

Dihydroterpineol (Menthanol) Aroma/Flavor Compound High Purity
$12.00 – $32.00 Select options This product has multiple variants. The options may be chosen on the product page -

Allyl Amyl Glycolate | Premium Fragrance/Aroma Compound
$16.00 – $29.99 Select options This product has multiple variants. The options may be chosen on the product page -

B-Caryophyllene Oxide | Natural Food Grade
$12.00 – $19.99 Select options This product has multiple variants. The options may be chosen on the product page
SKU: N/A
Categories: Fragrance Chemical, Fuel Additive, Solvents (3), Water Treatment
Tags: 120ml, Flammable Triethylamine, High-Purity Triethylamine, Industrial-Grade Triethylamine, Laboratory-Grade Triethylamine, Purified, TEA chemical, Triethylamine, triethylamine acidity, triethylamine adhesives, triethylamine agrochemical, triethylamine Alibaba, triethylamine Amazon, triethylamine amine, triethylamine analytical reagent, triethylamine API intermediate, triethylamine applications, triethylamine aroma compound, triethylamine B2B, triethylamine basicity, triethylamine beverage industry, triethylamine biodegradable, triethylamine boiling point, triethylamine bulk, Triethylamine Bulk Supplier, Triethylamine CAS 121-44-8, Triethylamine Catalyst, triethylamine chemical, triethylamine chemical distributor, triethylamine chemical engineering, triethylamine chemical exports, triethylamine chemical imports, triethylamine chemical plant, triethylamine chemical properties, triethylamine chemical research, triethylamine chemical synthesis, triethylamine chromatography, triethylamine cleaning products, triethylamine coatings, triethylamine compliance, triethylamine conditioner, triethylamine corrosion inhibitor, triethylamine cosmetics, triethylamine demand, triethylamine density, triethylamine deodorants, triethylamine detergents, triethylamine disinfectants, triethylamine distributor, triethylamine dyes, triethylamine e-commerce, triethylamine eBay, triethylamine eco-friendly substitutes, triethylamine elastomer catalyst, triethylamine emergency response, triethylamine emerging markets, triethylamine environmental impact, triethylamine environmental safety, triethylamine EPA, triethylamine evaporation rate, triethylamine explosive, triethylamine exposure limits, triethylamine factory, triethylamine FCC, triethylamine fine chemical, triethylamine first aid, triethylamine flammability, triethylamine flash point, triethylamine flavoring agent, triethylamine food grade, Triethylamine for Adhesive Production, Triethylamine for Agrochemicals, Triethylamine for Chemical Manufacturing, Triethylamine for Corrosion Inhibitors, Triethylamine for Dyes and Pigments, Triethylamine for Epoxy Resins, Triethylamine for Industrial Applications, Triethylamine for Laboratory Applications, Triethylamine for Organic Synthesis, Triethylamine for Paints and Coatings, Triethylamine for Petrochemicals, Triethylamine for Pharmaceuticals, Triethylamine for Polymer Production, Triethylamine for R&D, Triethylamine for Textiles, triethylamine formula, triethylamine fragrance, triethylamine fuel additives, triethylamine GC analysis, triethylamine GHS classification, triethylamine global market, triethylamine green chemistry, triethylamine hair care, triethylamine handling, triethylamine hazard statements, triethylamine hazards, triethylamine herbicide, triethylamine household chemicals, triethylamine HPLC, triethylamine import export, triethylamine industrial supply, triethylamine industrial use, triethylamine industry trends, triethylamine innovations, triethylamine intermediate, triethylamine international trade, triethylamine inventory, triethylamine lab chemical, triethylamine lab synthesis, triethylamine leather industry, triethylamine manufacturer, triethylamine marketplace, triethylamine melting point, triethylamine metal treatment, triethylamine molecular formula, triethylamine molecular weight, triethylamine MSDS, triethylamine new applications, triethylamine nitrogen compound, triethylamine oil refining, triethylamine online supplier, triethylamine organic base, triethylamine organic synthesis, triethylamine OSHA, triethylamine paints, triethylamine patents, triethylamine perfumes, triethylamine personal care, triethylamine pesticide, triethylamine petrochemicals, triethylamine pH, triethylamine pharma grade, triethylamine pharmaceutical use, triethylamine physical properties, triethylamine pigments, triethylamine plant, triethylamine plastics, triethylamine poison control, triethylamine polymerization catalyst, triethylamine precautions, triethylamine price, triethylamine procurement, triethylamine production, triethylamine production facility, triethylamine protective equipment, triethylamine quaternary ammonium salt, triethylamine raw material, triethylamine REACH, triethylamine reaction, triethylamine reagent, triethylamine regulations, triethylamine regulatory compliance, triethylamine research chemical, triethylamine resins, triethylamine rubber industry, triethylamine safety data, triethylamine sanitizers, triethylamine SDS, triethylamine sealants, triethylamine shampoo, triethylamine shipping, triethylamine skincare, triethylamine solubility, Triethylamine Solvent, triethylamine sourcing, triethylamine specialty chemical, triethylamine spill control, triethylamine storage, triethylamine structure, triethylamine supplier, triethylamine surfactants, triethylamine sustainable alternatives, triethylamine synthesis, triethylamine synthetic route, triethylamine TEA, triethylamine textile industry, triethylamine toxicity, triethylamine trade, triethylamine transportation, triethylamine uses, triethylamine vapors, triethylamine ventilation, triethylamine Walmart, triethylamine water treatment, triethylamine wholesale






Reviews
There are no reviews yet.